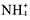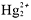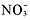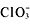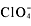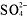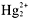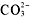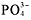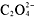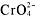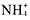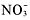Well... here are your solubility rules... sothat can get you an idea of what some of your products are... and theproducts are just what is on the other side of the ------>(probably called something diff in the US than Can?)
Ok so I found this online..
- Most alkali metal compounds and
compounds are soluble.
- Cl[suP]-[/suP], Br[suP]-[/suP], I[suP]-[/suP] compounds aresoluble, except when they contain Ag[suP]+[/suP],
,or Pb[suP]2+[/suP].
- F[suP]-[/suP] compounds are soluble, except when they contain group 2A metals.
-
,
,
,and CH[suB]3[/suB]COO[suP]-[/suP] compounds are soluble.
-
compounds are soluble, except when they include Ca[suP]2+[/suP],Sr[suP]2+[/suP], Ba[suP]2+[/suP], Ag[suP]+[/suP], Pb[suP]2+[/suP], or
.
-
,
,
,
,S[suP]2-[/suP], OH[suP]-[/suP], and O[suP]2-[/suP] compounds areinsoluble.
- Group 2A metal oxides are classified as strong bases even thoughthey are not very soluble.
The two solubility rules that youwill use the most are numbers 1 and 4. You must memorize that all group1A metal and ammonium compounds are soluble. As soon as you see acompound
,Li, Na, K, Rb, Cs, or Fr, you should know that its soluble. Also, allnitrates are solublelook at the end of the compound. If it ends in
,you know that its soluble.
Whats the big deal with solubility? Well, if the ion is soluble, itwont form a precipitate, and this means it doesnt react and should beleft out of the net ionic equation. The key is first to write thecompounds chemical formula and then determine if its soluble. If itis soluble, then ionize itif it isnt, dont ionize it; leave it as amolecule.
Edit: My last edit was wrong!! Ok... I'll think about it.. but this is all I have so far.

I don't know if this helps at all, and I may be wrong... soo... take it all with apound of salt!!
_________
Nadia